Assay for AAV Functional Titration (TCID 50 with qPCR)
Introduction
AAV (adeno-associated virus) functional titration assays are crucial for quantifying the functional viral particles in your AAV preparations. Functional titration measures the ability of AAVs to transduce cells and express the transgene, which is a more relevant parameter than genome titration when assessing viral potency.
A modified TCID50 assay is currently used to determine the infectious titer of recombinant adeno-associated virus (rAAV) using HeLaRC32 cells. In this system, the Rep and Cap proteins expressed by HeLaRC32 cells, along with the presence of wild-type adenovirus 5 (Ad5), enable the replication of AAV genomes within the cells. Real-time PCR is then employed to sensitively and quantitatively detect positive genome replication, providing an accurate measure of infectious titer.
At WZ Biosciences, a TCID50 assay has been developed with the following enhancements:
1. An in HEK293 cells replication-deficient adenovirus 5 (Ad5) is used instead of wild-type Ad5, minimizing complications related to Ad5 replication and reduced cell cytopathy.
2. HEK293 cells stably expressing Rep and AAVR are utilized to enable accurate infectious titer estimation across various AAV serotypes.
Service Details
WZ Biosciences offer following AAV TCID50 related products and services:
|
Cat No.
|
Products or Services
|
Specification
|
Price
|
Order
|
|
WZ-2G09
|
HEK293-2G09 Cell line
|
1 vial of 1.0E+6 HEK293 cells stable expression AAV2 Rep and AAVR gene.
|
$4995
|
In stock
|
|
AD-203002
|
Ad5d-GFP-Cre
|
In HEK293 cell replication deficient Ad5d-GFP-Cre
≥1.0*10E08 TU/ml, 50ul
|
$495
|
In stock
|
|
WZ140001-R
|
AAV infection unit estimation service
|
Estimation AAV infection unit comparing to standard AAV.
|
$195
|
1 week
|
|
WZ140001-A
|
Accurate AAV TCID50 service
|
Full scale of AAV TCID50 assay and report
|
$2995
|
3-4 weeks
|
*Interested in AAV TCID50 products and services? Please contact us “Request a quote”.
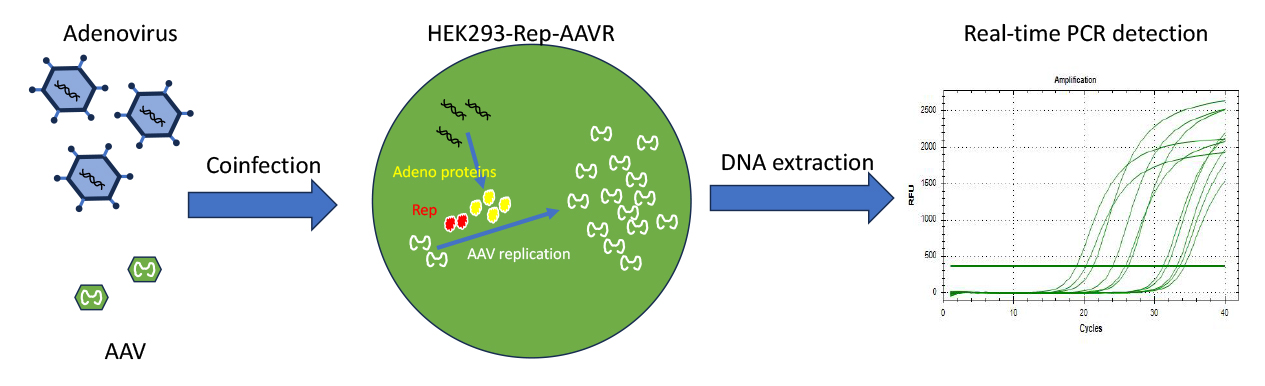
The Principle of AAV infection titer estimation and TCID50 assay
This AAV infection titer estimation and TCID50 assay determine the quantity of infectious viral particles capable of entering target cells and initiating transgene expression. This is a functional measure of AAV quality, assessing its ability to effectively transduce cells rather than just quantifying viral genomes or capsids.
If an AAV infects and gets into the nucleus of a permissive cells, with the presence of Rep protein and adenovirus, it’s genome can be replicated several thousand times. Only then the presence of AAV genome can be accurately detected by real-time PCR. This is the foundation of AAV functional titer or TCID50 assay.
The Tissue Culture Infectious Dose 50% (TCID50) assay estimates the infectious titer of AAV by determining the viral concentration at which 50% of cell culture wells show a positive infection signal (e.g., genome replication). This method uses statistical analysis to calculate the titer based on the observed infection pattern across serial dilutions.
The schematic diagram of AAV TCID50 assay is blow:
The schematic diagram of AAV TCID50 assay
Key Features:
1. In-HEK293 cell replication deficient Ad5d-GFP-Cre adenovirus instead of wild type Ad5 eliminates complications associated with adenovirus replication and cytopathic effects, ensuring a more controlled assay environment.
- The ability to use a high multiplicity of infection (MOI) (up to 10) with this replication-deficient adenovirus significantly enhances assay sensitivity by promoting efficient helper activity for AAV genome replication and transgene expression.
- Extending the assay duration to 72 hours further improves consistency and reproducibility by allowing sufficient time for complete viral processes, including genome replication.
2. The stable expression of Rep and AAVR in HEK293 cells ensures accurate infectious titer estimation across a wide range of AAV serotypes.
- Rep facilitates efficient AAV genome replication.
- AAVR, a universal receptor for multiple AAV serotypes, enhances viral entry.
- The combination creates a standardized cellular environment that minimizes variability and enables consistent transduction efficiency, regardless of the AAV serotype being tested.
Service Process
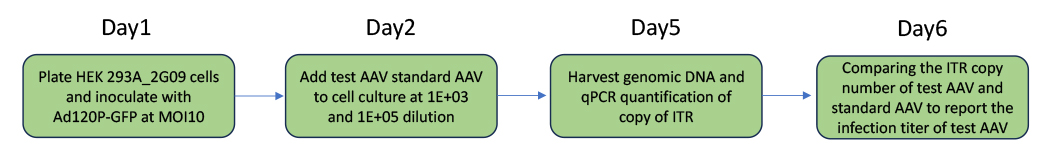
1. AAV infection unit estimation service
This service is only provided with our AAV packaging services. With this service, we guarantee the AAVs produced by WZ Biosciences have infection titer no less than 1.0E+11IU/ml for AAV serotyope 2 and 1.0E+10IU/ml for other major AAV serotypes and genome titer is higher than 1.0E13GC/ml.
The schematic Diagram for services process of AAV infection unit estimation service
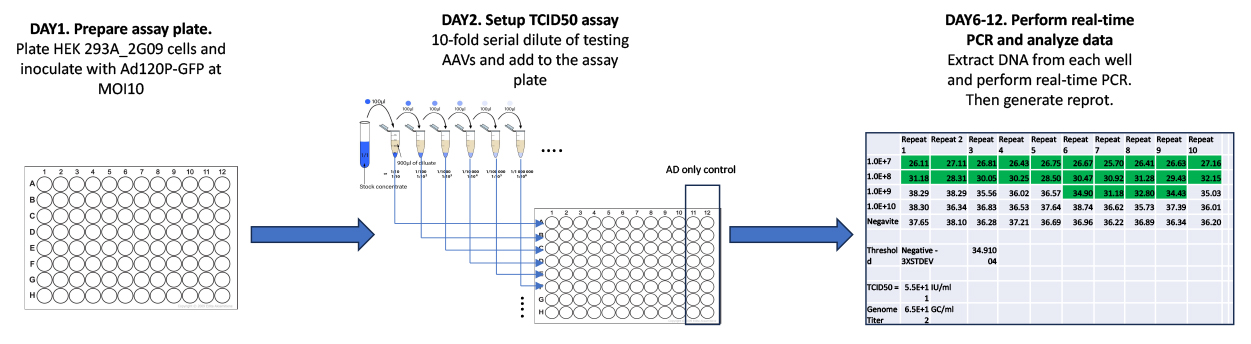
2. Accurate AAV TCID50 service
This service is for customers who need accurate TCID50 titer of the AAV virus for research and therapeutic purpose. As little as 10ul AAV at genomic titer >1.0E1+13 form the customer is sufficient for complete the assay.
The schematic Diagram for services process of Accurate AAV TCID50 service
Related AAV QC services
AAV titer by ddPCR AAV infection titer by TCID50 AAV Capsid purity by SDS PAGE AAV Capsid Empty/Full by AEX HPLC rcAAV contamination.
FAQs
The AAV TCID50 (Tissue Culture Infectious Dose 50) assay is commonly used to determine the infectious titer of adeno-associated viruses (AAV). Here’s a list of frequently asked questions (FAQs) regarding this assay:
1. What is the purpose of the TCID50 assay for AAV?
The assay measures the number of infectious AAV particles in a sample by determining the dose required to infect 50% of a cell culture. This provides an estimate of the biologically active viral particles.
2. How does the TCID50 assay work?
When an AAV infects and gets into the nucleus of a permissive cells, with the presence of Rep protein and adenovirus, it’s genome can be replicated several thousand times. Only then the presence of AAV genome can be accurately detected by real-time PCR.
3. What are the key improvements in WZ Biosciences for better TCID50 assay?
· Cell Line Selection: WZ Biosciences created HEK293-2G09 Cells express an AAV receptor, which are thousand times more permissive to most AAV serotype being tested.
· Transduction Conditions: WZ Biosciences’ in HEK293 cells replication deficient Adenovirus enable more flexible in Adenovirus and AAV transduction, such as the MOI selection and incubation duration.
· Reporter System: In our system, the AAV genome can be replicated several thousand times, thus it is easier and more reliable to detection of AAV genome copy number by real-time PCR.
4. What is the culture condition for HEK293-2G09 cells?
HEK293-2G09 cells can grow in DMEM medium supplement with 10%FBS. The integrated Rep and AAV receptor gene may not very stable. For long term use, you can always purchase from WZ Biosciences Inc.
5. Can I reamplify Ad5d-GFP-Cre adenovirus from WZ Biosciences Inc.?
Our Ad5d-GFP-Cre adenovirus is in HEK293 cell replication deficient. You should not try to reamplify it in HEK293 cells. It can be purchased from WZ Biosciences Inc.
6. Do I need MTA for using Ad5d-GFP-Cre adenovirus and HEK293-2G09 cells from WZ Biosciences Inc.?
Yes. You need to get MTA from WZ Biosciences Inc. before you purchase and use them. For MTA please contact techsupport@wzbioscience.com.
Protocol
Thawing Frozen Cells
1) Take the cryovial containing the frozen HEK293-2G09 cells from the package or liquid nitrogen storage and immediately place it into a 37°C water bath.
2) Quickly thaw the cells (< 1 minute) by gently swirling the vial in the 37°C water bath until there is just a small bit of ice left in the vial.
3) Wipe the outside of the vial with 70% ethanol and transfer the vial into a laminar flowhood.
4) Transfer the cells into a 10cm culture plate with 10ml pre-warmed DMEM medium with 10%FBS and 1% PS.
5) 6 to 12hrs after, when the HEK293-2G09 cells were settled down then change the medium with 10ml pre-warmed DMEM medium with 10%FBS and 1% PS.
6) In 48 hrs, HEK293-2G09 cells should be full in the plate.
Infecting HEK293-2G09 with Ad5d-Cre-GFP adenovirus
1) Disassociate the HEK293-2G09 cells, and then resuspend the cells with complete growth medium with density at 6.0E+05 viable cells per ml of total 6 ml.
2) Add 6E+06 IFU Ad5d-Cre-GFP per ml of total of 15ul of 1.0E+08 IFU Ad5d-Cre-GFP adenovirus to 6ml HEK293-2G09 cells.
3) Add mixture of HEK293-2G09 cells and Ad5d-Cre-GFP adenovirus to 96 well cell culture plates at 50ul per well.
4) return the plates to cell culture incubator and culture for 24hrs.
Setup serial dilution of testing rAAV in full cell culture medium (With rAAV2 genome titer around 1E+13gc/ml as an example)
1) Prepare 10X1.5ml Eppendorf tubes with 900ul full cell culture medium.
2) Dilute the virus as follows: Mix dilutions thoroughly,
Dilution 1 (100X): add 10 µL of AAV with 90ul culture medium to the first 1.5ml Eppendorf tube.
Dilution 2 (1000X): 100 µL from Dilution 1 to 900uL culture medium
Dilution 3 (1.0E+4X): 100 µL from Dilution 2 to 900uL culture medium
Dilution 4 (1.0E+5X): 100 µL from Dilution 3 to 900uL culture medium
Dilution 5 (1.0E+6X): 100 µL from Dilution 4 to 900uL culture medium
Dilution 6 (1.0E+7X): 100 µL from Dilution 5 to 900uL culture medium
Dilution 7 (1.0E+8X): 100 µL from Dilution 6 to 900uL culture medium
Dilution 8 (1.0E+9X): 100 µL from Dilution 8 to 900uL culture medium
Dilution 9 (1.0E+10X): 100 µL from Dilution 9 to 900uL culture medium
Setup AAV TCID50 Assay (With rAAV2 genome titer around 1E+13gc/ml as an example)
1) Check the infection of HEK293-2G09 cells with Ad5d-Cre-GFP adenovirus in 96 well plate. Under microscope, check all the wells in the 96 well plate. An almost complete and uniform expression of GFP should be expected. No cell death or detachment of cells should be expected.
2) Add 50ul of testing rAAV2 dilutions at dilution of 7 to 10 to 96 wells plate. Each dilutions are added to each row with 10 replicates from well 1 to well 10.
3) Add 50ul of medium to well 11 and well 12at each row as the adenovirus only control.
4) Setup Row A to D and Row E to H as duplications.
5) Return the 96 well plates to cell culture incubator for 72hrs.
Genomic DNA extraction and real-time PCR assay
1) Check again the infection of HEK293-2G09 cells with Ad5d-Cre-GFP adenovirus in 96 well plate. Under microscope, check all the wells in the 96 well plate. An almost complete and uniform expression of GFP should be expected. No cell death or detachment of cells should be expected.
2) Add digestion buffer 20ul 10X Proteinase K buffer (1% SDS, 10mM EDTA, 10mM PBS ph8.0) with final 25ug proteinase K/well.
3) Incubate at 37degree 1hr.
4) Check again the 96 well plate. Under microscope, check all the wells in the 96 well plate. A completely lysed cells should be expected. No patch of cells should be expected.
5) Transfer all solution (about 120ul) to a 96 well PCR plate, at 95degree for 10min to inactive proteinase K.
6) Prepare a new 96 well PCR plate, added 90ul ddH2O to each well.
7) Transfer 10ul cell lysate from each well to above new 96 wells with 90ul ddH2O. This results 10X dilution of the cell lysate and is read for real-time PCR assay.
Setup real-time PCR Assay and analyze the data
1) Perform real-time PCR assay with your selected system with 2ul sample from each well.
2) After Real-time PCR, calculate the negative threshold= Average of CT of all the negative wells -3X standard derivation
3) For All the samples, if CT value is less than the threshold, the sample is considered as positive.
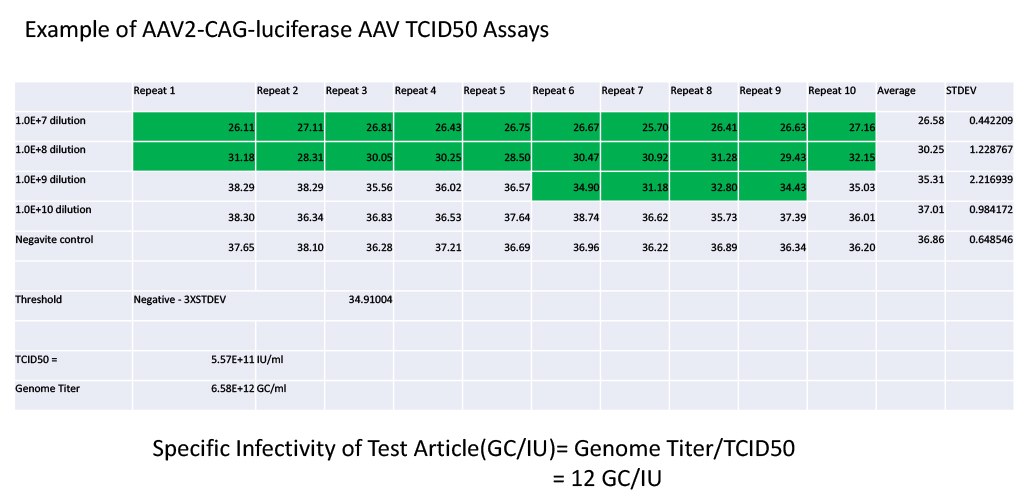
Example and TCID50 calculation
Green high-lighted wells are considered as positive wells.
|
4)
|
Replicate1
|
Replicate2
|
Replicate3
|
Replicate4
|
Replicate5
|
Replicate6
|
Replicate7
|
Replicate8
|
Replicate9
|
Replicate10
|
Average
|
SD
|
Average-3XSD
|
|
NT
|
37.89
|
34.95
|
36.37
|
35.00
|
35.82
|
35.05
|
35.38
|
37.88
|
34.89
|
34.73
|
35.80
|
1.2
|
32
|
|
1.0E07
|
28.14
|
27.42
|
28.49
|
28.98
|
29.14
|
28.78
|
28.23
|
28.00
|
26.54
|
26.54
|
28.03
|
0.9
|
|
|
1.0E08
|
31.81
|
27.87
|
29.53
|
31.20
|
31.87
|
32.97
|
31.58
|
30.88
|
32.22
|
31.35
|
31.13
|
1.4
|
|
|
1.0E09
|
38.63
|
36.45
|
34.87
|
33.29
|
36.29
|
31.00
|
35.30
|
33.31
|
35.12
|
34.19
|
34.85
|
2.1
|
|
|
1.0E010
|
35.95
|
34.91
|
35.52
|
30.45
|
31.30
|
35.56
|
36.01
|
35.71
|
35.82
|
35.62
|
34.69
|
2.0
|
|
Infection Titer (IU/mL) = E(L+S-0.5)*V
L = 7
S = 1+8/10+1/10+2/10= 2.1
V= 120ul/50ul/2ulX1000=1200
Infection Titer = E (7+2.1-0.5) X1200= E(8.6)X1200=4.78E11 IU/mL
Examples
1. TCID50 measurement of AA2-CAG-Luciferase AAV produced by WZ Biosciences Inc.
The infection titer of AAV2-CAG-luciferase AAV with genomic titer of 6.58E+12GC/ml was assayed with Ad5-GFP-Cre adenovirus in HEK293-2G09 cells following our AAV TCID50 protocol. The ratio of genome titer vs that of Infection titer is about 12GC/IU.
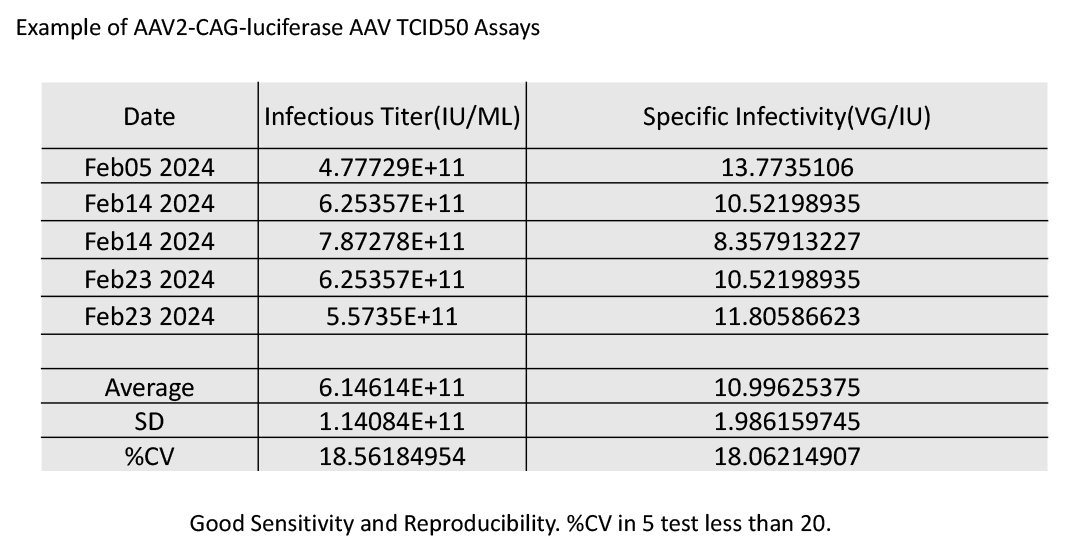
2. 5 repeats of the TCID50 measurement of AA2-CAG-Luciferase AAV produced by WZ Biosciences Inc. Our data shows the consistence of our measurement. The %CV in our 5 tests is less than 20.
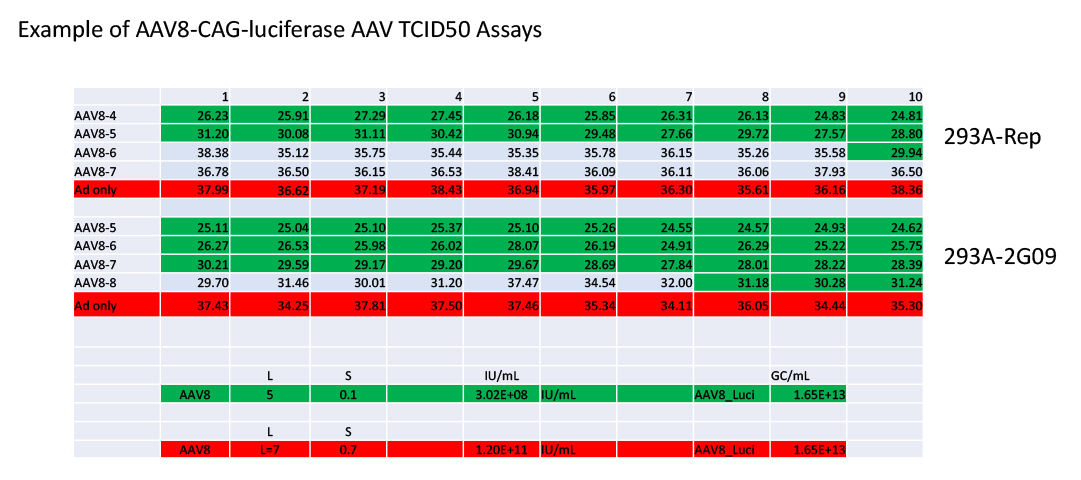
3. Comparing of the TCID50 measurement of AA8-CAG-Luciferase AAV produced by WZ Biosciences Inc in a 293-Rep cell lines vs in HEK293-2G09 cells. In 293 cells, AAV8 shows significantly lower transduction efficiency when comparing that of AAV2. Thus, a very low TCID50 measurement of AAV8-CAG-Luciferase AAV is expected. Our data showed it is about 1000 times lower than AAV2-CAG-Lucifease AAV when the genome titers are the same. While, when HEK293-2G09 is used, the TCID50 of AAV8-CAG-Luciferase was improved about 300 times.